It sounds like magic. Drop a droplet on a soap bubble and it stretches into the bubble in a flower shape. Melika Motaghian was surprised at first, but now she can explain it physically. It doesn’t make the phenomenon any less eye-catching or insightful.
Melika Motaghian (Physics and Physical Chemistry of Foods) is doing her PhD on the spreading behaviour of liquids. Understanding and controlling this behaviour is important for the development of products such as shampoo, lubricants, and detergents. The Marangoni effect plays an important role in her research. You can see this physical phenomenon in a glass of strong wine: the wine tears.
Bubbles
The tears are caused by a difference in surface tension between water and alcohol (ethanol) in the wine. Water has a higher surface tension than ethanol. At the edges of the glass, the ethanol evaporates more quickly and therefore there is more water. With the higher surface tension, the water pulls at the liquid around it: the wine rises, until it falls back due to gravity.
Motaghian uses the Marangoni effect to test the spreading behaviour of liquids in a bubble. She examined mixtures of surfactants, like soap, and polymers – for example, polyethylene glycol, a molecule with a long chain of ethylene glycol. First, she stretched a soap bubble in a special device, the scientific version of a bubble blowing ring. Then she drops one drop of the mixture on it. The drop does not fall on or through the soap film, it falls into it. Because the soap has a higher surface tension, it stretches the drop.
Daisies in rainbow colours
At low concentrations of the polymer, the drop stretches like a perfect circle in the soap bubble. This happens in a few tens of milliseconds. At higher concentrations, the liquid becomes more viscous and the drop stretches more slowly. It then takes a few hundred milliseconds. ‘The surprising thing is that above a certain concentration the drop no longer stretches like a circle, but like a flower of rainbow colours, a daisy pattern,’ says Motaghian. She published her discovery in the Journal of Colloid and Interface Science.
The daisy is created by the elasticity of the polymers in the droplet, Motaghian discovered. Above a certain concentration, the long polymer strands become entangled with each other. That makes the liquid elastic. So when the drop falls into the bubble, it does not stretch equally everywhere. These fluctuations in stretching look like the petals of a daisy. The rainbow colours are due to the refraction of light, explains Motaghian: ‘During stretching, the thickness of the droplet decreases from the centre to the edge, and that thickness is a factor of the wavelengths of visible light. Therefore, a different wavelength – and therefore colour – of light refracts and reflects each time.’
Stable
Motaghian’s research is fundamental physics but has many applications. The daisy, the instability in stretching behaviour, is undesirable. ‘You want a shampoo that completely covers your hair, or a detergent that reaches an entire surface,’ she explains. ‘That’s why I’m looking into how this instability arises, and how you can stabilise the stretching behaviour.’

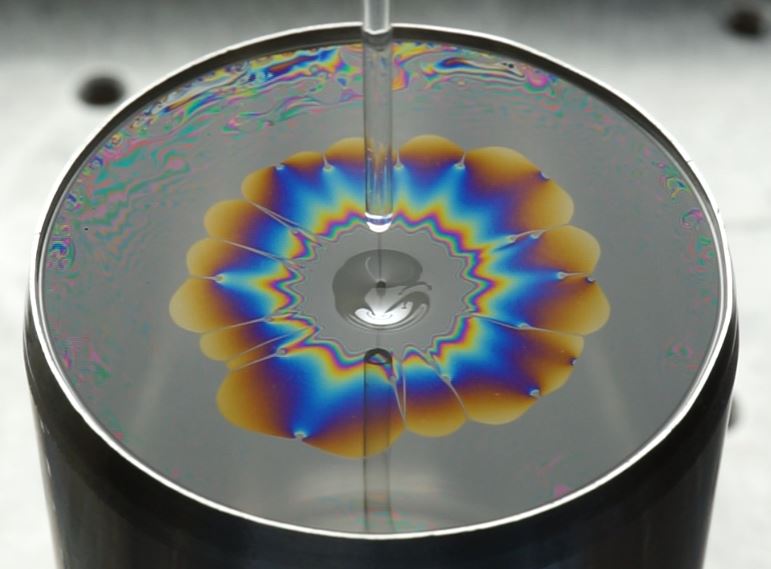 A droplet of polymer-surfactant solution spreading on a soap film. When the concentration of polymer in the droplet is higher than a critical level, the spreading front of the droplet destabilises into a daisy shape pattern. Images are half a second apart. Photo by Melika Motaghian.
A droplet of polymer-surfactant solution spreading on a soap film. When the concentration of polymer in the droplet is higher than a critical level, the spreading front of the droplet destabilises into a daisy shape pattern. Images are half a second apart. Photo by Melika Motaghian.